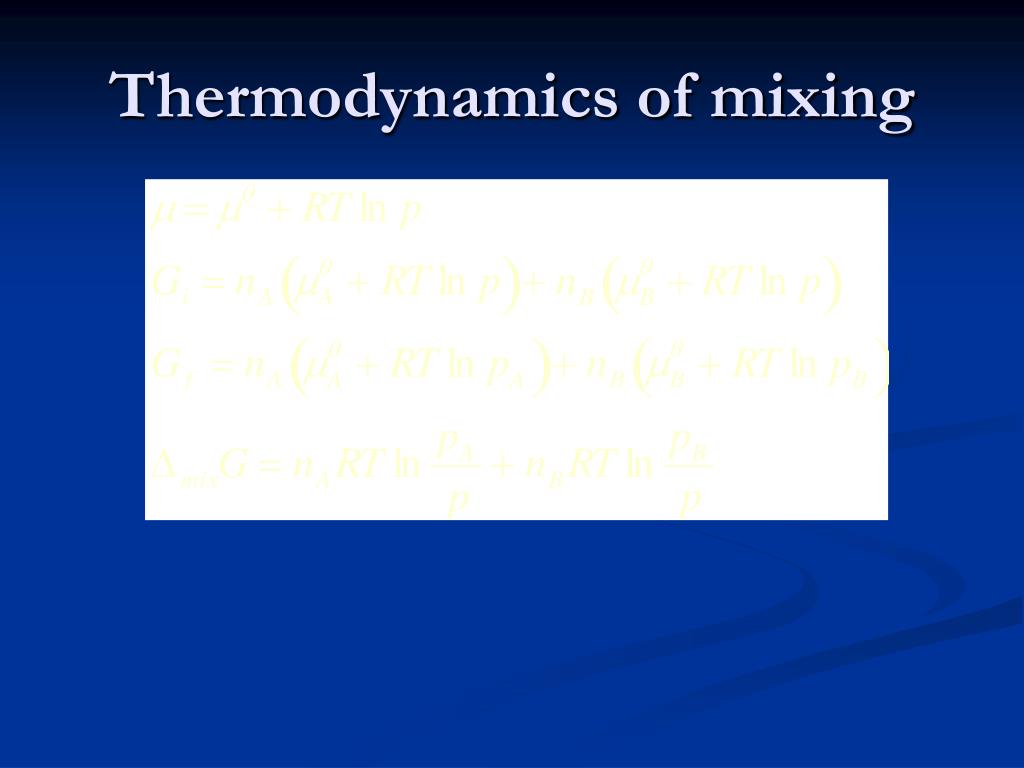
Mixing Rule Thermodynamics . When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. This module will discuss the effect that mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each.
from www.slideserve.com
Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing.
PPT SIMPLE MIXTURES PowerPoint Presentation, free download ID258447
Mixing Rule Thermodynamics We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. This module will discuss the effect that mixing. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations.
From chem.libretexts.org
13.2 Phase Diagrams Binary Systems Chemistry LibreTexts Mixing Rule Thermodynamics Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. Mixing rules are used to obtain numerical estimates for the parameters in an equation of. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT Chapter 7 Simple Mixtures PowerPoint Presentation, free download Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. We can use the experimental result that (for similar liquids) the mole fraction of the substance. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT Equation of State Modeling of Phase Equilibrium in the Low Mixing Rule Thermodynamics Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. This module will discuss the effect that mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a. Mixing Rule Thermodynamics.
From www.youtube.com
Lecture 33 Mixing Thermodynamics. YouTube Mixing Rule Thermodynamics This module will discuss the effect that mixing. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT SIMPLE MIXTURES PowerPoint Presentation, free download ID258447 Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. When solids,. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT SIMPLE MIXTURES PowerPoint Presentation, free download ID258447 Mixing Rule Thermodynamics Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. Mixing rules allow the coupling of both types of. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT SIMPLE MIXTURES thermodynamic description of mixtures PowerPoint Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. Mixing rules are used to obtain numerical estimates for the parameters in an equation of. Mixing Rule Thermodynamics.
From slideplayer.com
C = 2 Gibbs phase rule F = C P + 2 Pressure Temp. Comp. xA F = 3 Mixing Rule Thermodynamics Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. When solids, liquids or gases are combined, the thermodynamic. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT SIMPLE MIXTURES PowerPoint Presentation, free download ID258447 Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. Mixing rules are used to obtain numerical estimates for the parameters in an equation of. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT Professor Douglas A. Loy Visiting from the University of Arizona Mixing Rule Thermodynamics Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. When solids, liquids or gases are combined, the thermodynamic quantities of. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT Short Version 18. Heat, Work, & First Law of Thermodynamics Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. Mixing rules are used to obtain numerical estimates. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT The thermodynamic description of mixtures Partial molar Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. We can. Mixing Rule Thermodynamics.
From sciencenotes.org
Laws of Thermodynamics Mixing Rule Thermodynamics This module will discuss the effect that mixing. Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. When solids, liquids. Mixing Rule Thermodynamics.
From www.youtube.com
Thermodynamics Determining Fugacity in a Vapor Mixture Using Equations Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. This module will discuss the effect that mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. Mixing rules allow the coupling of both types. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT The thermodynamic description of mixtures Partial molar Mixing Rule Thermodynamics We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. When solids, liquids or. Mixing Rule Thermodynamics.
From www.researchgate.net
(PDF) Thermodynamics of mixtures Functions of mixing and excess functions Mixing Rule Thermodynamics Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. This module. Mixing Rule Thermodynamics.
From www.youtube.com
11.02 Entropy Change of Mixing and Ideal Solutions YouTube Mixing Rule Thermodynamics Mixing rules allow the coupling of both types of thermodynamics models to overcome such limitations. This module will discuss the effect that mixing. When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. We can use the experimental result that (for similar liquids) the mole fraction of the. Mixing Rule Thermodynamics.
From www.slideserve.com
PPT Equation of State Modeling of Phase Equilibrium in the Low Mixing Rule Thermodynamics When solids, liquids or gases are combined, the thermodynamic quantities of the system experience a change as a result of the mixing. Mixing rules are used to obtain numerical estimates for the parameters in an equation of state for a specified mixture from. This module will discuss the effect that mixing. When solids, liquids or gases are combined, the thermodynamic. Mixing Rule Thermodynamics.